Saturday, 2026-01-31, 10:06 AM | Welcome Guest | Registration | Login
Bridge to Health - Live your Life in Harmony with Nature
Publisher
| Main » Articles » My articles |
Magnesium and Magnesium Deficiency
 Magnesium is a chemical element with the symbol Mg, atomic number 12 and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust, where it constitutes about 2% by mass, and ninth in the known Universe as a whole. This preponderance of magnesium is related to the fact that it is easily built up in supernova stars from a sequential addition of three helium nuclei to carbon (which in turn is made from three helium nuclei). Magnesium ion's high solubility in water helps ensure that it is the third most abundant element dissolved in seawater. Magnesium is a chemical element with the symbol Mg, atomic number 12 and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust, where it constitutes about 2% by mass, and ninth in the known Universe as a whole. This preponderance of magnesium is related to the fact that it is easily built up in supernova stars from a sequential addition of three helium nuclei to carbon (which in turn is made from three helium nuclei). Magnesium ion's high solubility in water helps ensure that it is the third most abundant element dissolved in seawater. Magnesium is the 11th most abundant element by mass in the human body; its ions are essential to all living cells, where they play a major role in manipulating important biological polyphosphate compounds like ATP, DNA, and RNA. Hundreds of enzymes thus require magnesium ions to function. Magnesium is also the metallic ion at the center of chlorophyll, and is thus a common additive to fertilizers. Magnesium compounds are used medicinally as common laxatives, antacids (i.e., milk of magnesia), and in a number of situations where stabilization of abnormal nerve excitation and blood vessel spasm is required (i.e., to treat eclampsia). Magnesium ions are sour to the taste, and in low concentrations help to impart a natural tartness to fresh mineral waters.
Magnesium is a vital component of a healthyhuman diet. Human magnesium deficiency (including conditions that show few overt symptoms) is relatively common, with only 32% of the United States meeting the RDA-DRI; low levels of magnesium in the body has been associated with the development of a number of human illnesses such as asthma, diabetes, and osteoporosis. Adult human bodies contain about 24 grams of magnesium, with 60% in the skeleton, 39% intracellular (20% in skeletal muscle), and 1% extracellular. Serum levels are typically 0.7 – 1.0 mmol/L or 1.8 - 2.4mEq/L. Serum magnesium levels may appear normal even in cases of underlying intracellular deficiency, although no known mechanism maintains a homeostatic level in the blood other than renal excretion of high blood levels. Intracellular magnesium is correlated with intracellular potassium.
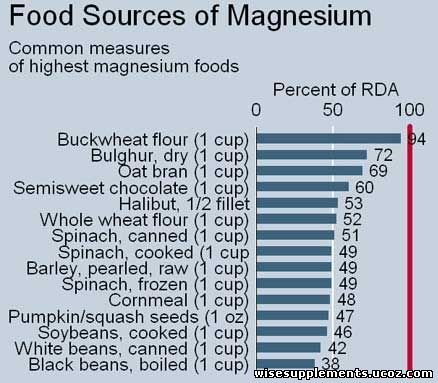 Magnesium is absorbed in the gastrointestinal tract, with more absorbed when status is lower. In humans, magnesium appears to facilitate calcium absorption. Low and high protein intake inhibit magnesium absorption, and other factors such as phosphate, phytate, and fat affect absorption. Absorbed dietary magnesium is largely excreted through the urine, although most magnesium "administered orally" is excreted through the feces. Magnesium is absorbed in the gastrointestinal tract, with more absorbed when status is lower. In humans, magnesium appears to facilitate calcium absorption. Low and high protein intake inhibit magnesium absorption, and other factors such as phosphate, phytate, and fat affect absorption. Absorbed dietary magnesium is largely excreted through the urine, although most magnesium "administered orally" is excreted through the feces. Magnesium status may be assessed roughly through serum and erythrocyte Mg concentrations and urinary and fecal excretion, but intravenous magnesium loading tests are likely the most accurate and practical in most people. In these tests, magnesium is injected intravenously; a retention of 20% or more indicates deficiency. Other nutrient deficiencies are identified through biomarkers, but none are established for magnesium.
Spices, nuts, cereals, coffee, cocoa, tea, and vegetables are rich sources of magnesium.
Green leafy vegetables such as spinach are also rich in magnesium as they contain chlorophyll, which is rich in magnesium.
Observations of reduced dietary magnesium intake in modern Western countries compared to earlier generations may be related to food refining and modern fertilizers that contain no magnesium.
| |
| Views: 1160 | Rating: 0.0/0 |
| Total comments: 0 | |

